Stress Analysis Of A Cardiovascular Stent
A manufacturer of cardiovascular stents developed a new design that required FDA approval before it could be sold in the United States. In order to obtain this approval it had to be shown that the stent could withstand the balloon expansion process without developing any cracks. Also, it had to be shown that the implant could withstand the long-term in-vivo cyclic fatigue loading created by blood pressure pulsation. To this end, MSI was commissioned to perform a finite element based computer simulation of the stent performance.
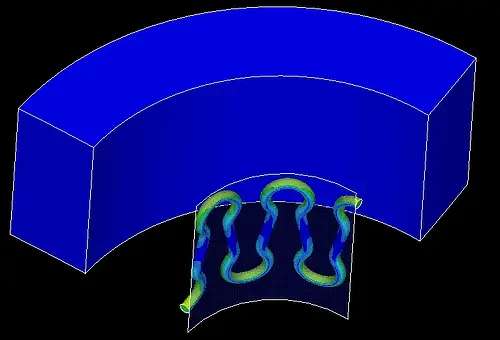
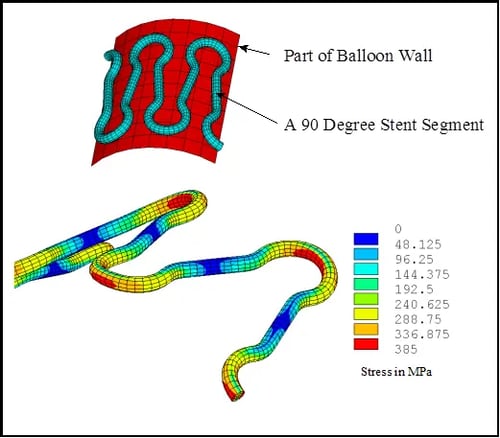
Stress analysis of a cardiovascular stent is quite complex due to its highly nonlinear behavior. During the balloon expansion process it is common for the stent diameter to increase by more than a factor of three. By design, significant plastic deformation occurs within the implant so that it does not spring back to its original shape after balloon deployment. Since the deployment stresses are in the plastic regime, the implant does not spring back, but rather holds an artery open in an expanded state. MSI incorporated this plastic behavior of the stainless steel implant into the finite element analysis.
The complete behavior of the stent was simulated in the finite element analysis. The balloon was modeled as well as the artery. Contact elements were placed between the balloon and the implant as well as between the implant and the arterial wall. The balloon was made to expand so that the implant was forced to a diameter slightly greater than that of the artery. The balloon was then deflated and the plastically deformed implant continued to hold the artery open. A pulsating blood pressure was then applied to the artery and the implant stress history was evaluated.
The calculated mean and alternating stress found within the implant were placed on a Goodman Diagram for the material in order to assess the fatigue life. It was found that this implant should withstand many millions of blood pressure pulsations without fatigue failure. Also, the peak strains during balloon expansion were low enough so that no fracture would be expected during deployment. The results were found to be in agreement with physical testing performed by the manufacturer. This work led to an increased confidence level in the structural integrity of the implant and was convincing enough to the FDA to allow clinical trials to proceed.
REAL-WORLD EXAMPLES AND CASE STUDIES
MSI In Action
Case Study
Improving Cost of Ownership with Vibration Risk Reduction: 1/2
Focusing on vibration and dynamics issues during the plant design phase of a project pays off with smoother commissioning and lower cost of ownership over the plant’s life.
Case Study
Simulating the Performance of a Spinal Implant
A manufacturer of an innovative spinal implant approached MSI for help in assessing the implant’s structural integrity.
Case Study
Dynamic Analysis of a Street Sweeper for the Manufacturer
Troubleshoot and identify potential opportunities for early bearing failure, MSI performed experimental modal analysis (EMA) and operational deflection shape (ODS) tests on a prototype unit for the manufacturer.